Drs Mekhail and Hu awarded CIHR grants
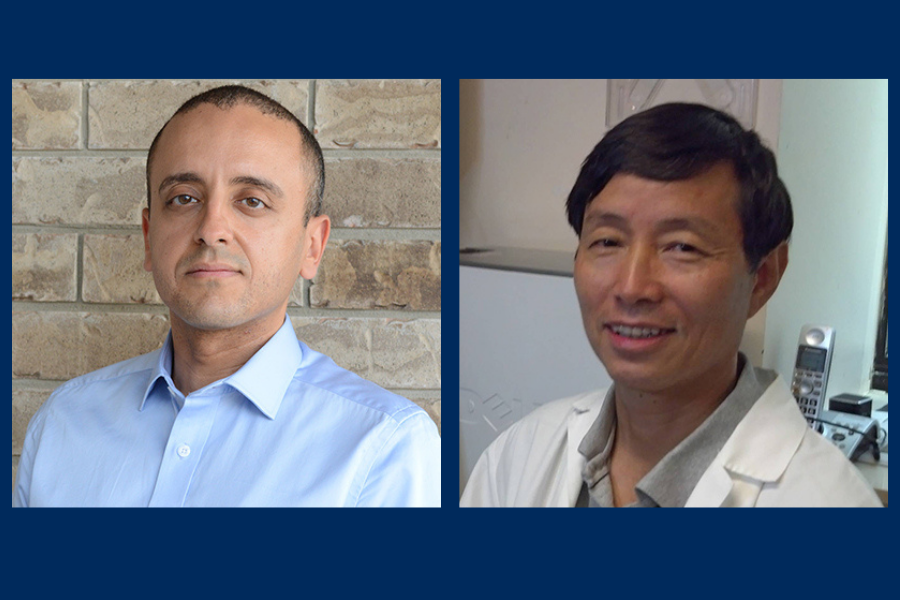
We are delighted to announce that two primary-appointed faculty and four cross-appointees in the Department of Laboratory Medicine and Pathobiology have been awarded funding in the latest Canadian Institutes of Health Research (CIHR) round.
Congratulations to all!
Bi-locus gene replacement for enhancing permanent correction of genetic airway diseases
Principal investigator: Dr. Jim Hu
Co-investigators: Dr. Amy Wong and Dr. Hartmut Grasemann
The goal of this project is to test a novel strategy for achieving therapeutic levels of gene correction in patient cells and animal models, using cystic fibrosis (CF) as a model.
CF is the most common fatal disease in the Caucasian population and is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Currently, lung disease with respiratory failure due to chronic infection and inflammation is causative for most CF related morbidity and mortality. Recent progress in the discovery of drugs that target the CFTR protein directly leads to effective therapies for the first time in most patients, providing the proof of concept that restoration of CFTR function in patients' cells is a practical approach for treating cystic fibrosis. Although the current new drug therapy is a milestone in bringing clinical benefits to CF patients, there is a small portion (10%) of CF patients that cannot be covered by the therapy. In addition, daily drug burden for patients has not been reduced and potential long-term side effects are not clear. Therefore, new therapeutic options are needed.
We are developing an alternative strategy to integrate a functional CFTR gene into the genome of affected cells. The human airways are readily accessible to gene delivery vehicles, but previous gene therapy lacks the delivery efficiency and sustained therapeutic gene expression. Recent advancements in gene editing technology presents unprecedented opportunity for advancing gene therapy. Our published work has shown the feasibility of integrating the functional CFTR gene in cultured CF cells. The current proposal is to test a strategy by targeting more than one genetic locus and by further enhancing the gene targeting efficiency. We will test the gene targeting efficiency and therapeutic benefits in patient primary cells and in CF ferrets. We will also test a combined gene and drug therapy in attempt to lower the drug dose and expand drug benefits to more patients.
Control of genome stability by a nanotubular nuclear envelope network
Principal investigator: Dr. Karim Mekhail
Co-investigator: Dr. Razq Hakem
Our human body comprises trillions of cells whose DNA is packaged inside a balloon-like nuclear envelope and can be damaged by various factors such as smoking and unsafe exposure to the sun. Therefore, the survival and health of our cells depend on processes that recognize and repair damaged DNA. Notably, the human nuclear envelope somehow ensures the repair of damaged DNA, even if most of the damaged DNA appears far from the nucleus's edge. So, how the envelope promotes human DNA repair remains a mystery.
Here we pursue new findings suggesting that in cells with damaged DNA, rod-like structures called microtubules poke the exterior of the balloon-like nuclear envelope at multiple points, creating a network of finger-like nuclear envelope invaginations. These finger-like invaginations may contact damaged DNA and drive its repair by sticking the broken DNA ends to each other. The invaginations are omnipresent in breast cancer cells suggesting an increased reliance on these finger-like structures to repair accumulating DNA damage. We have two main objectives.
First, we will decipher how the remodeled nuclear envelope mediates DNA repair to ensure cell survival. Second, we will study whether the increased reliance of breast cancer cells on these invaginations for DNA repair and cell survival can be exploited to fight cancer.
Overall, this work will uncover critical health-related knowledge about DNA repair, cell survival, and growth while revealing how it can be applied to combat deadly human diseases.
Cross-appointed faculty in LMP
The following cross-appointed faculty also received funding in this round:
- Dr. Peter Dirks (Department of Surgery)
Determining the cellular mechanisms of initiation and evolution of hypermutant glioma - Dr. Rod Bremner (Department of Ophthalmology & Vision Sciences)
Dissecting the Tumor Suppressor Function of YAP in Neural and Neuroendocrine Cancers - Dr. Carol Schuurmans (Department of Biochemistry)
Targeting Pten to unlock the regenerative potential of the mammalian retina - Dr. Ana Konvalinka (Department of Medicine)
Determining Donor-Specific Antibody Pathogenicity in Kidney Transplantation Using Tissue Proteomics and Systems Immunology