From lab to patent: an innovative approach to support heart attack recovery

Heart disease is the number one cause of death globally. According to the Heart and Stroke Foundation of Canada, one person dies in Canada every five minutes from a heart condition. The most common manifestation of heart disease is a blocked artery which causes a heart attack.
Dr. Jonah Burke-Kleinman recently completed his PhD in the Department of Laboratory Medicine and Pathobiology under the supervision of Dr. Michelle Bendeck (LMP) and Dr. Paul Santerre (Faculty of Dentistry and Institute of Biomedical Engineering). The investigators are part of the Translational Biology and Engineering Program within the Ted Rogers Centre for Heart Research. During his PhD, Dr. Burke-Kleinman and the team developed a treatment that could improve outcomes for heart attack patients who had been treated with angioplasty, and the team has filed a patent for the discovery in the hope it can be commercialized to reach patients.
“The most common treatment for a blocked artery that causes a heart attack is a balloon angioplasty. They guide a catheter up from the femoral artery to where it's blocked, and inflate it to push aside the blockage, similar to snaking a drain like a plumber,” explains Burke-Kleinman.
This treatment is very effective at reopening the artery but injures the cells. This can cause restenosis, a process where the cells from the walls migrate and accumulate matrix-forming tissue in the middle of the artery.
Patients often receive drug-eluting stents to combat this - a metal fence cylinder put inside the artery which holds it open and releases a drug that kills the cells around it, therefore preventing cells from moving and re-blocking the artery. “This is the gold-standard therapy, and it works well, but the drugs used are indiscriminately cytotoxic. They kill not only the type of cell we want to stop, which is the smooth muscle cells but also a good type of cell, the endothelial cell. Endothelial cells are critical for making a non-stick surface along the interior of arteries. Where you are lacking in them, you get clots”, Jonah explains.
The Bendeck Lab had previously discovered that the smooth muscle cells received their cue of when and where to move from a protein called N-Cadherin. N-Cadherin is present where the cell touches another, but absent where there is a gap, so the cell moves into the space. “It’s as if the cell is asking if it has a neighbour.”
Having studied endothelial cells during his master’s at Queen’s, he moved back to Toronto, where he grew up, and joined the Bendeck Lab to explore these cells further.
The team developed a short piece of protein, called a peptide, that could mimic N-Cadherin which then engages N-Cadherin on the smooth muscle cell, tricking it into thinking it has a neighbour and can’t move.

“Endothelial cells don't depend on N-Cadherin for their movement. When we applied the peptide, it slowed the migration of the smooth muscle cells but not endothelial cells. In our animal studies with rats, it reduced the amount of thickening in the wall of the artery after injury. This made it a good candidate for a cell-type selective therapy.”
This work was recently published in the journal Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB) with one of Burke-Kleinman’s images featured on the cover. Matrix-Binding, N-Cadherin–Targeting Chimeric Peptide Inhibits Intimal Thickening but Not Endothelial Repair in Balloon-Injured Carotid Arteries.
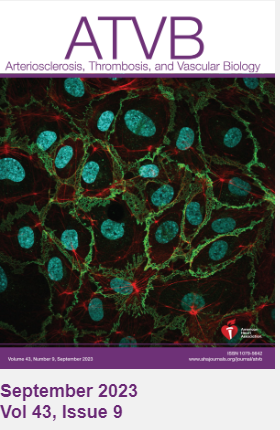
Burke-Kleinman was proud to have his image on the cover of ATVB. His images have become a hit within the Department of LMP, as he has been successful in the annual LMP Art Competition – coming 2nd place in 2022 and winning the 2023 competition. “It's an exciting way to share our images with people. I think seeing is believing when it comes to science. A lot of what we do is microscopic and can be hard to explain and understand, even for scientists in the field. If you can produce a clear image and see in detail what you're studying, what the cell looks like and what it's doing, it goes a long way to helping understand and communicate our research”.
The team worked with the Innovations and Partnerships Office at the University of Toronto to put together a disclosure on the discovery and then file a provisional and a full US patent. “This was a vital step, if we hope this will one day become a therapy and reach patients. Investors, pharmaceutical or medical device companies will be more interested in commercialising research if there is concrete Intellectual Property protection behind it.”
When asked if he plans to be involved in the commercialization of the discovery, Burke-Kleinman laughs, “It can be very difficult and unpredictable. I've thought about taking our invention and trying to make a new company with it, but after being in school for so many years I'd like to get more industry experience first. Maybe later if I want to pursue something entrepreneurial, I will.”
In the meantime, he is working as a postdoctoral fellow with the Santerre lab while he builds connections for his career. “I would love to work in consulting or venture capital as somebody who can explain science to investors, and advise whether to invest in science startups. Translating research from the bench to the bedside is a big challenge but comes with potentially big rewards.”
This story showcases the following pillars of the LMP strategic plan: Dynamic Collaboration (pillar 2), Impactful Research (pillar 3), and Agile Education (pillar 5)



